Are you seeking for 'write a balanced equation for the complete combustion of cyclopentane'? All material can be found on this website.
What is the counterpoised chemical equation for the complete burning of cyclopentane? C5H10 + O2 = CO2 + Water – Chemical Equivalence Balancer.
Table of contents
- Write a balanced equation for the complete combustion of cyclopentane in 2021
- C5h10 + o2 product
- Combustion of cyclopentane equation
- C5h10+o2=co2+h2o balanced equation
- What is the coefficient for oxygen when balancing the complete combustion of cyclopentane c7h10
- C5h10 + o2 reaction
- Incomplete combustion of cyclopentane
- C5h10 refrigerant
Write a balanced equation for the complete combustion of cyclopentane in 2021
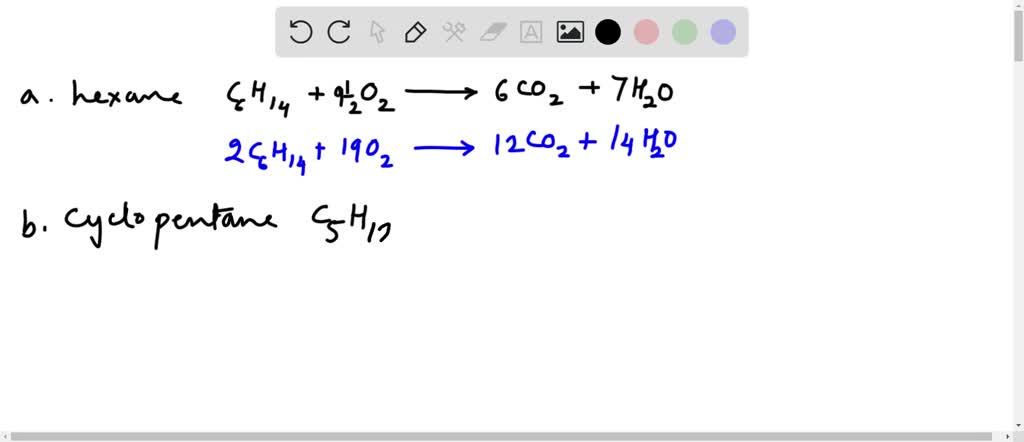 This picture representes write a balanced equation for the complete combustion of cyclopentane.
This picture representes write a balanced equation for the complete combustion of cyclopentane.
C5h10 + o2 product
 This image shows C5h10 + o2 product.
This image shows C5h10 + o2 product.
Combustion of cyclopentane equation
 This picture representes Combustion of cyclopentane equation.
This picture representes Combustion of cyclopentane equation.
C5h10+o2=co2+h2o balanced equation
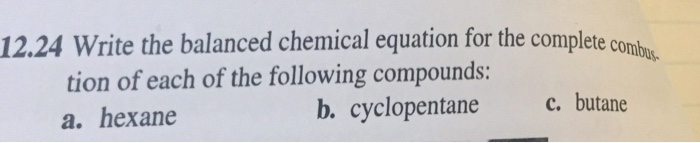 This image demonstrates C5h10+o2=co2+h2o balanced equation.
This image demonstrates C5h10+o2=co2+h2o balanced equation.
What is the coefficient for oxygen when balancing the complete combustion of cyclopentane c7h10
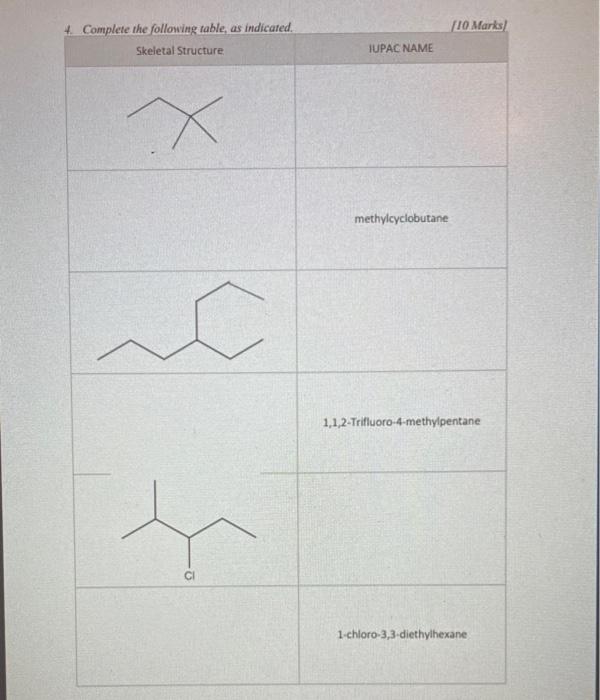 This image illustrates What is the coefficient for oxygen when balancing the complete combustion of cyclopentane c7h10.
This image illustrates What is the coefficient for oxygen when balancing the complete combustion of cyclopentane c7h10.
C5h10 + o2 reaction
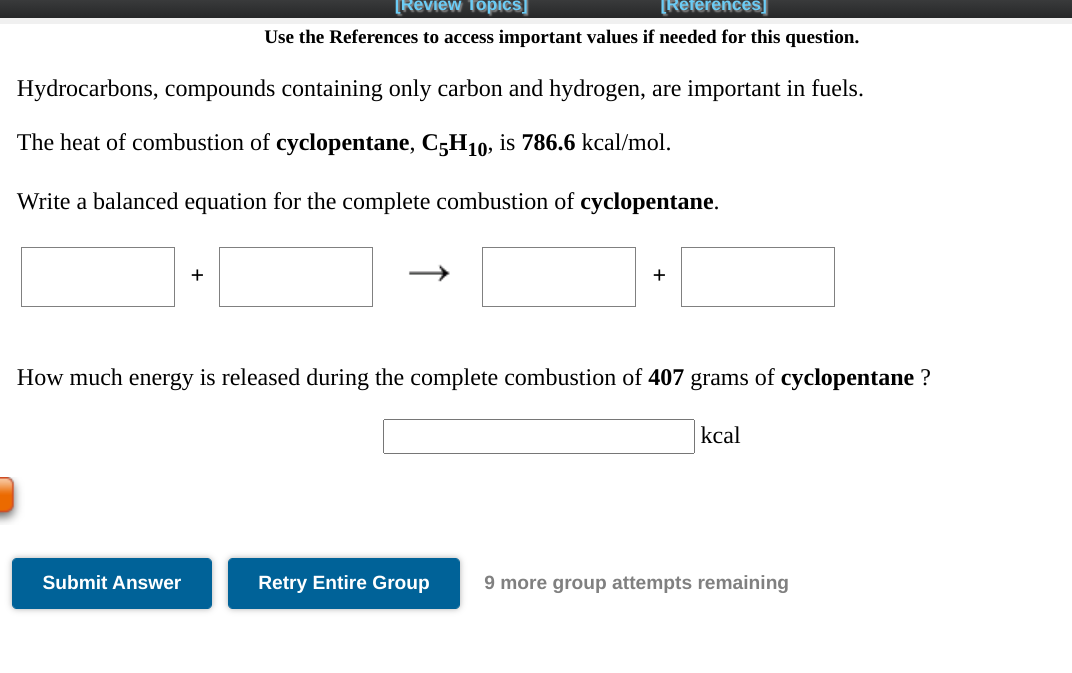 This image demonstrates C5h10 + o2 reaction.
This image demonstrates C5h10 + o2 reaction.
Incomplete combustion of cyclopentane
 This image shows Incomplete combustion of cyclopentane.
This image shows Incomplete combustion of cyclopentane.
C5h10 refrigerant
 This image demonstrates C5h10 refrigerant.
This image demonstrates C5h10 refrigerant.
When does a valid combustion reaction take place?
Valid combustion reaction is an exothermic reaction which occur when oxygen react with another substance . It commonly occur when hydrocarbons burns completely in presence of oxygen to form H2O and CO2. Hydrocarbon + O2 → H2O + CO2. Is C6H6 O2 → CO2 H2O balanced? The equation is now balanced. What is the balanced equation of C6H6 O2?
What happens when Cyclopentane is completely combusted?
Being a hydrocarbon, cyclopentane, when completely combusted, produces CO2 & H2O. when Oxygen supply is limited, incomplete combustion occurs, and the products can contain carbon monoxide (CO) gas, and H2O vapour.
What is the balanced equation for the incomplete combustion of?
Equations (2) & (3) show the effect of gradually decreasing amount of O2 gas, on the combustion of cyclopentane. ie, incomplete combustion. Note: The degree of oxidation of carbon in the cyclopentane, C5H10, molecule decreases as the ratio of C5H10 : O2 decreases. (See the equations 1,2&3 above).
What is the balanced chemical equation for C5H10 O2?
C5H10 + O2 = CO2 + H2O – Chemical Equation Balancer. What is the balanced equation for C5H10 O2 CO2 H2O?
Last Update: Oct 2021
Leave a reply
Comments
Evylene
28.10.2021 08:26Hydrocarbons: hydrocarbons are natural science compounds formed aside the bonding betwixt hydrogen and C atoms.